Abschnitt 2.2 - 2.2 Molten metals
Molten magnesium has a viscosity comparable to that of water in the casting temperature range and ignites spontaneously on contact with atmospheric oxygen. Technical magnesium alloys melt at temperatures in the range of approx. 420 °C to 650 °C (self-ignition from approx. 400 °C, most alloys melt from 530 °C).
Without covering agents or protective gases, the molten metal ignites in the air and immediately burns on the surface with a strong development of white smoke and bright white light.
The resulting harmful smoke consists of magnesium oxide with a particle size of < 10 µm. This smoke should not be inhaled and falls within the scope of the workplace limit for the alveolar dust fraction (TRGS 900).
A distinctive feature of magnesium fire is the glaringly bright and, due to the heat radiation in the infrared range, blinding flame. In addition, UV/IR and visible portions of radiation in the flame can occur. Unprotected viewing can cause damage to the eyes (e. g. cataract). A visor, for example with gold coating, should be used.
When the molten metal comes into contact with moisture, water or even rust etc., a violent reaction takes place, in the course of which molten metal is ejected. This can lead to subsequent fires, causing personal injury or damage to property. It is therefore essential to prevent contact of the molten metal with water and moisture!
The high combustion temperatures of magnesium of up to 3000 °C result in a splitting into oxygen and hydrogen (thermolysis) when water is added. This can lead to an explosive oxyhydrogen gas reaction with potentially fatal consequences.

Figure 15
Ingot casting/production of ingots
Activities/protective measures
Inserting ingots
When ingots are inserted, any ingress of moisture must be prevented. The ingots must be dried to avoid adhering moisture, for example by preheating stations (temperature and minimum time as specified by the manufacturer). Careful insertion of the magnesium ingots by hand or through a sluice into the metal bath (e. g. through a sliding duct, suitable baskets) protects the crucible from damage.
Gassing/protective gases
When opening crucible flaps and inserting ingots, sensors, pumps and similar, there is always the risk of atmospheric oxygen and traces of moisture ingressing the system.
In order to prevent this, protective gas is applied at overpressure. Any disturbance of the molten bath and the associated access of air should be kept as short as possible. Otherwise, turbulences might be caused by convection, so that air can enter and displace the protective gas.
The magnesium oxidizes at the surface of the molten metal with the oxygen in the air to form magnesium oxide. The magnesium oxide is unstable (oxygen diffuses), has a smaller volume than the molten magnesium and thus causes the layer above the molten metal to break up again and again. Accordingly, spontaneous combustion at the surface may occur. To prevent that from happening, a protective gas is applied to the surface of the molten metal. Different gases can be used for this purpose. Currently used protective gas atmospheres consist of the following gases, for example: R 134a or SO2 (a few percent) mixed with absolutely dry air or pure nitrogen.
Skimming/crucible cleaning
During melting or holding, even under protective gas, granular slag is formed on the bath surface of magnesium crucibles through oxidation. This so-called dross forms a heterogeneous mass which must be regularly removed (skimming).

Figure 16
Dross container made of steel ...

Figure 17
... with tight-fitting cover

Figure 18
Container for skimming tools, open

Figure 19
Container for skimming tools, closed

Figure 20
Foundry clothing worn on the oven platform during skimming (flame-resistant silver
clothing, respiratory protection if required, gold-plated visor)
The molten magnesium adhering when the dross is removed ignites very quickly in the air outside the crucible, producing smoke. Therefore, the molten magnesium and the tool should be placed in a completely dry, non-combustible suitable container (e. g. made of steel) with a tightly closing lid, in which the fire is quickly extinguished. Alternatively, the fire can also be extinguished by covering it with a special melting salt.
The tools used to clean the crucible should also be stored in suitable containers or kept clean and dry to prevent the residues adhering to them from igniting.
Dross from non-soluble oxides of the alloy components occurs primarily on the the molten bath surface, but also at the bottom and walls of the crucible. It must also be removed or "pulled" regularly. Dross/scale deposits must also be removed at regular intervals from the side walls and the bottom of the crucible.
The removal of dross from the surface or of oxides from the crucible may only be carried out under the following conditions.
Suitable protective clothing is worn (e. g. flame-resistant "silver clothing", if necessary, respiratory protection, gold-coated visor). See Section 3 "Personal protectiveequipment".
Preheated tools, specially designed for the operating conditions are used. The tools must be made of solid material, be dry and free from rust, nickel and impurities.
It must be prevented that, when opening the crucible flaps, possible caking from oxides generated during the melting process fall into the molten metal in larger lumps (danger of thermite reaction Mg/Fe2O3). A possible protective measure is the regular inspection and cleaning.
The deposits at the bottom and walls of the crucible (bottom slag) must be removed at regular intervals, as overheating can occur in these areas and lead to crucible rupture.
Extraction (pull out) and cleaning of the pump
A fire caused by adhering molten metal can also occur when metal pumps, stirrer motors or temperature probes are pulled out. Such fires can also be extinguished by covering them with thick layers of silicate-free mineral wool on the basis of magnesium and calcium oxide. As soon as no more oxygen reaches such a manageable fire and the magnesium solidifies, the flames are extinguished.
If the pump or the pouring container is pulled out, there is a risk of ignition of the adhering, partly liquid, magnesium. Therefore, the components should be placed quickly in a steel container with cover.
In practice, the component is covered with suitable covering/extinguishing agents (e. g. ceramic fiber wool/dry sand/hollow glass granulate (silicon dioxide)) and the cover of the container is closed in order to extinguish the flames. In addition, suitable extinguishing agents of fire class D should be available in sufficient quantities for these activities.
Melting furnace/crucible
When using protective gases containing fluorine, a coating of the inside of the crucible at liquid level with nickel-free chromium steel to increase corrosion resistance has proven to be advantageous.
Linings in the crucible area must be made of refractory material, free from silicates and iron oxide, since molten magnesium can react with these oxides. The brick lining/collecting tray should preferably be made of steel or of a material with a high alumina content.
A temperature setting as uniform as possible and monitoring with several temperature sensors in the different sections of a crucible to avoid major variations is required. In addition, the liquid level of the crucible must be monitored with level probes. The liquid level should be kept constant so that especially the upper edge area of the crucible is not overstressed.
A routine monitoring of the crucible wall thickness by ultrasonic or other non-destructive methods must be carried out as well as a visual inspection of the walls for cracks and deformation. According to previous experience, the service life of the crucibles is about 4-6 years, depending on the quality. However, this must be examined on a case-by-case basis. If the wall thickness is reduced, the specifications of the crucible or furnace manufacturer must be observed. In general, regular inspection intervals are to be determined by the user taking into account the manufacturer's information.
When using R134a as a protective gas, attention must be paid to the corrosive effect of hydrofluoric acid (HF) that inevitably forms in the area of the metal surface on the crucible edge. Regular inspection of the wall thickness of the crucible and its structural condition is also necessary.
Mechanical overstressing of the crucible by an excessively violent insertion of the ingots into the molten magnesium should be avoided. Slow insertion and melting of the ingots should be preferred.
It should be possible to separately disconnect the supply of the melting furnace with electrical energy, gas and hydraulic fluid outside the hazard zone. In an emergency, the supply of protective gas must be maintained. For this reason, casting cells generally need their own emergency power supply.

Figure 21
Die casting system
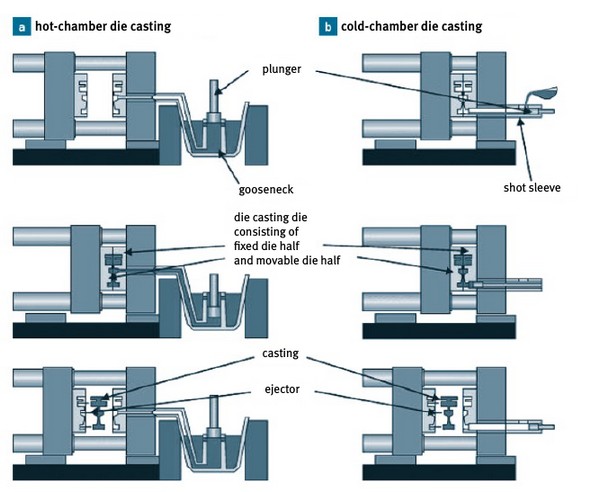
Figure 22
Schematic representation of the course of the die casting process: (a) hot chamber
die casting machine, (b) cold chamber die casting machine
