Abschnitt 1 - Introduction
| Small structures with special properties |
|---|
Nanotechnology is an important field for research and development, which is where nanomaterials are manufactured or processed. As defined in international standards, the key focus with nanotechnology is on the tiny structures (from approximately 1 nm to around 100 nm) in the context of the special properties caused by their tiny size (e. g. the extremely large surface area relative to the mass or also quantum mechanic effects). Nanomaterials differ from other common dangerous substances not only in terms of their chemical composition, but also because their complex spatial structure has a significantly further reaching influence. The influence of the typically very large specific surface is discussed. Highly reactive oxygen radicals may be created.
| Types of nanomaterials |
|---|
Nanomaterials include nano-objects in the form of nanoplates and nanofilms (e. g. graphene), nanotubes and nanorods (e. g. carbon nanotubes), as well as nanoparticles (e. g. fullerene or metal oxide particles). Composite nanomaterials which either contain nanoscop ic structures (e. g. embedded carbon nanotubes) or carry them on their surface (e. g. chips) are also classed as nanomaterials (Fig. 1).
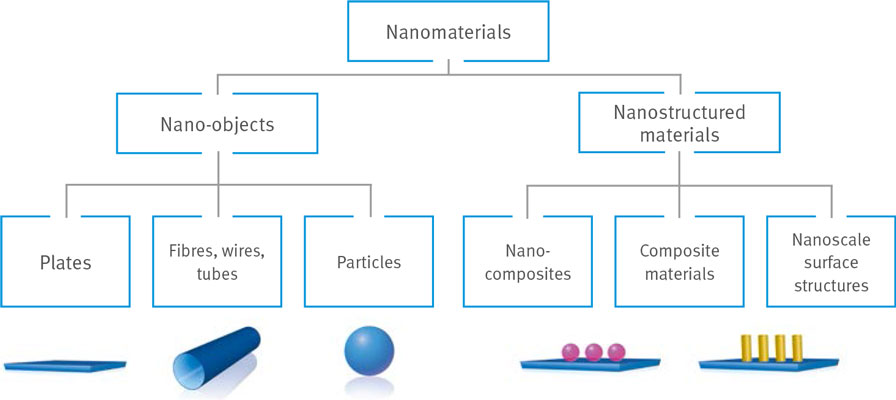
Knowledge of their potential hazards remains patchy to date. For example, some toxicological investigations performed indicate that several nano-objects might have negative effects on human health, while other investigations have been unable to confirm this. The fact that several nano-objects can penetrate biological structures is already known, yet whether this can also have a negative effect remains unclear. Some fibrous nanomaterials show worrying results in animal tests.
According to a recommendation by the European Commission, all materials produced or created in processes as well as natural materials that contain unbonded nano-objects ("particles") as agglomerates or aggregates in a numerical concentration of at least 50% (which can mean a very low weight ratio) with one or more external dimensions between 1 nm and 100 nm are to be designated as nano-materials. Where particular concerns exist, this content level can be lowered to 1%. Fullerenes, graphene flakes and single-walled carbon nanotubes with one or more external dimensions of less than 1 nm are also classified as nanomaterials. If the particle number concentration is not known, the material is classified as a nanomaterial if it has a specific surface of at least 60 m2/cm3.
In its Announcement No. 527 on Hazardous Substances, the Committee on Hazardous Substances of the Federal Ministry of Labour and Social Affairs (AGS) proposes classifying nanomaterials into four groups according to their assumed potential impact.
| I: | Soluble nanomaterials (solubility of at least 100 mg/l water under normal conditions) |
|---|---|
| II: | Biopersistent nanomaterials with specific toxicological properties |
| III: | Biopersistent nanomaterials without specific toxicological properties (GBP 1) nanomaterials) |
| IV: | Biopersistent, fibrous nanomaterials |
The AGS also proposes introducing concentration limits in the air at the workplace for the assessment. These limits are to be observed with the proven protective measures in laboratories under proper use.
| Risk of fire and explosion |
|---|
Combustible nano-objects are potentially explosive when mixed with air. Indeed, their ignitability can be much higher than more coarse-grained materials (significantly lower minimum ignition energy than dusts). Dangerous, explosive atmospheres can be created with relatively low quantities. As little as 100 mg of fine material (or even less) can be sufficient for ignition in unfavourable circumstances. Attention must therefore also be paid to ignition sources in equipment and apparatus. Some materials (such as metals) have a tendency to spontaneously combust in fine dispersions. The reactivity can be very high and catalytic effects are also possible.
| Release from bound structures |
|---|
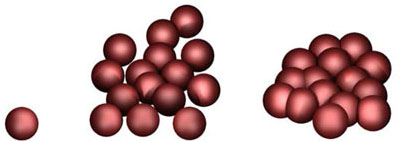
Nano-objects tend to join together to create larger units in the form of agglomerates (with weaker binding forces) or aggregates with relatively strong bonds (Fig. 2).
| Principle of precaution |
|---|
During processing, these units can cause free nano-objects to again be released in certain circumstances, for example through grinding in a (high-performance) mill. Nano-objects can also be released in certain circumstances when processing composite nanomaterials, e. g. when removing or breaking the matrix (for example by removing a solvent or by grinding a material with embedded nano-objects). Based on current knowledge, however, the nano-objects created in these processes seem to be formed from the destroyed material of the matrix in the systems investigated and do not appear to come from the embedded nano-objects.
A conclusive assessment of the risks cannot yet be performed based on the current level of knowledge. The principle of precaution therefore requires pragmatic solutions to be found for effective protective measures in lab work. One advantage here is that airborne nano-objects behave less like fine dusts and more like gases or vapours. This means that the standard protective measures for such material states in the lab can also be used for nanomaterials. However, free fibrous structures or those that release fibres (nanotubes, nanorods) should currently be handled with increased caution and care.
granular biopersistent particles
